Cytiva Original Medical Brand 29185230 Xuri T cell Expansion Medium 500ml
Support Payment Term:

HSBC Hong Kong

paypal

Alibaba Pay

Western Union

USD

EUR

GBP

SGD

HKD

CNH

CAD

MXN

BRL

JPY

THB

MOP

AUD

NZD

PLN

CZK

HUF

RON

CHF

SEK

NOK

DKK

TRY

AED

SAR

ILS

ZAR
$0
Discover similar items
Page 1 of 2
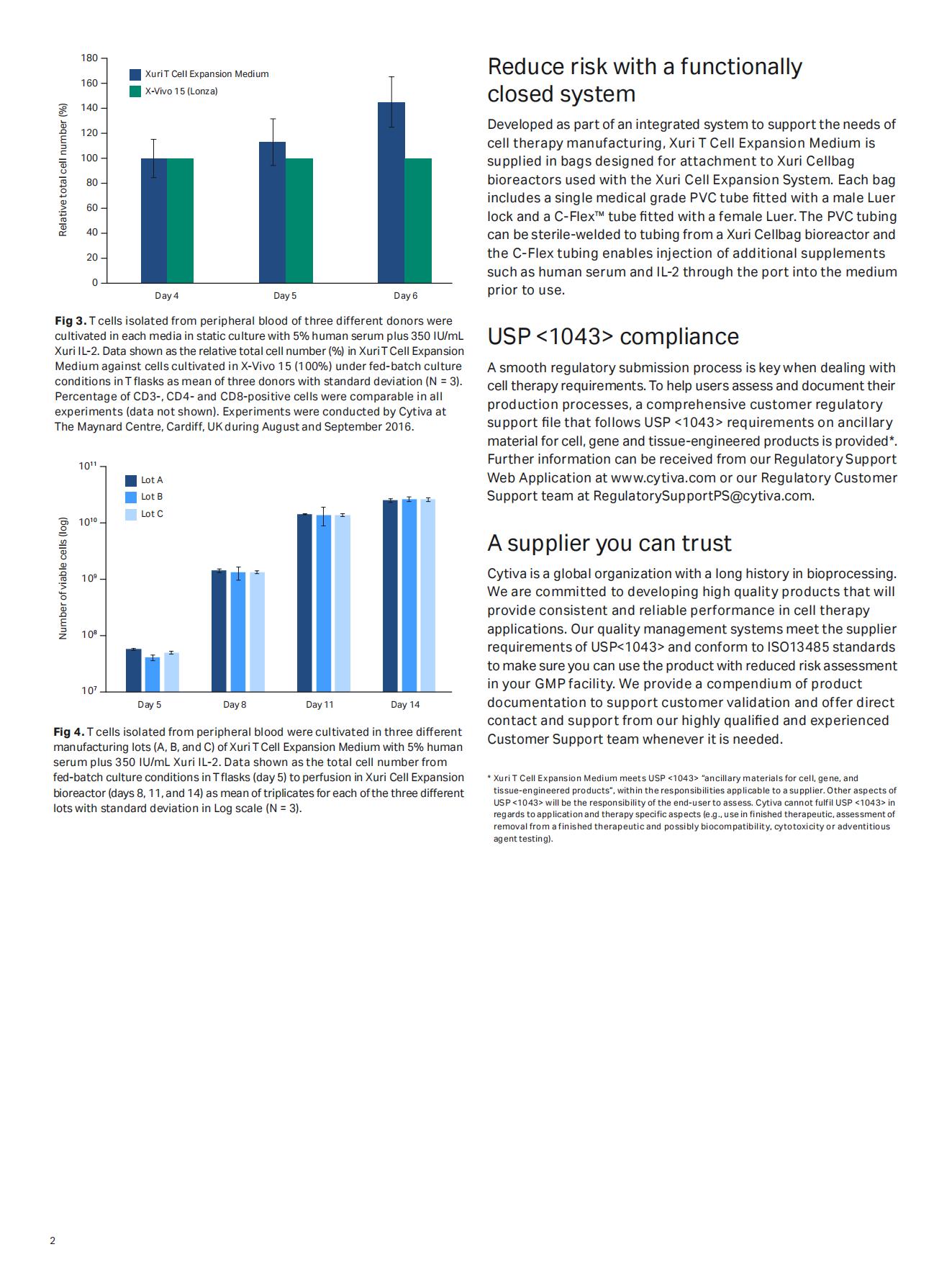
Rapid cell proliferation for faster manufacturing
Xuri T cell expansion medium enables elevated rates of proliferation of viable human T lymphocytes to high cell densities. Cultivation time becomes more flexible, enabling the reach of the desired cell number faster even when working with challenging cells due to donor variability.
Reduce risk with a functionally closed system
Xuri T cell expansion medium is shipped in bags equipped with medical grade PVC tubing and fitted with male and female Luer lock ports, making sure that the bag can be sterile welded to Xuri cellbag bioreactors. This provides the flexibility to add supplements to the bag within a functionally closed system.
Minimize variability for consistent performance
Xuri T cell expansion medium is manufactured under a strict quality management system and the fully validated production process ensures consistency across batches, improving control in achieving the desired cell numbers.
USP <1043> compliance
A smooth submission process is key when dealing with cell therapy regulatory requirements. To help users assess and document production processes, Cytiva supplies Xuri T cell expansion medium with a comprehensive documentation support package that meets USP <1043> ‘ancillary materials for cell, gene, and tissue-engineered products’, within the responsibilities applicable to a supplier.
End user responsibility
Other aspects of USP <1043> will be the responsibility of the end user to assess. Cytiva cannot fulfill USP <1043> in regard to application and therapy specific aspects (e.g., use in finished therapeutic, assessment of removal from a finished therapeutic and possibly biocompatibility, cytotoxicity or adventitious agent testing). The product is chemically defined and free from any human or animal derived components.